Stroke Diagnosis & Treatment

Hospitalization
Stroke hospitalization can be divided into acute and subacute phase. In the acute phase, urgent patient transfer to the hospital is essential to stabilize the patient and differentiate ischemic from hemorrhagic stroke through brain imaging, usually in the emergency department. In case of ischemic stroke, arrival in a stroke center during the first hours after symptom onset allows for acute recanalization therapies to reestablish brain flow in the ischemic part of the brain and potentially rescue brain tissue from irreversible damage. The short window of opportunity makes rapid transfer of stroke patients to the appropriate center critical[1].

Post Stroke
Unfortunately, only a small minority of stroke patients in Greece are currently admitted in specialized stroke units, capable of providing recanalization therapies (intravenous thrombolysis and mechanical thrombectomy) 24/7/365. According to the SITS registry[2], intravenous thrombolysis is offered in only 1% of ischemic stroke patients, and mechanical thrombectomy in less than 0.5% [4]. Even allowing for some unregistered cases, most Greek patients suffering from ischemic stroke and fulfilling criteria for acute recanalization treatments are deprived from modern stroke care.
Treatment Options & Delivery
Following acute treatment and stabilization of patient, main objectives of hospitalization in the subacute phase are to prevent medical complications and stroke recurrence. Deep vein thrombosis cause by immobility may lead to life-threatening pulmonary embolism, severe infections of the lungs or the urinary tract may progress to sepsis, threatening cardiopulmonary function [5]. Correct secondary stroke prevention treatment should be started in the following days after admission to prevent stroke recurrence that leads to extension of the brain lesions. Secondary stroke prevention treatment algorithms are similar in ischemic stroke and transient ischemic attack and should be tailored to each patient depending on comorbidities and the cause of the stroke7. Antithrombotic treatment is the cornerstone of secondary ischemic stroke prevention and consists of antiplatelet therapy (double in the subacute phase for selected patients and single in the chronic phase for most patients) in non-cardioembolic strokes and anticoagulation for cardioembolic strokes, in eligible patients[6]. Novel combined therapies are currently on clinical trials and may further reduce recurrence rates[7].
Atrial Fibrillation Diagnostic Options
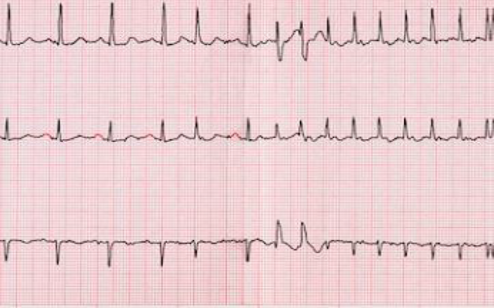
1. ECG
An ECG is a simple, painless test that records cardiac electrical activity. It is the most useful test for the diagnosis of atrial fibrillation. The ECG records the heart rate and also the heart rate (rhythmic or arrhythmic activity). However, the ECG records electrical activity for only a few seconds. Thus patients who at that time do not have atrial fibrillation cannot be immediately diagnosed. In patients with paroxysmal atrial fibrillation, which is intermittent in nature, the diagnosis can be made with continuous heart rate monitors (Holter).
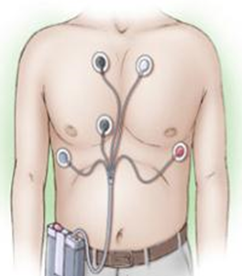
2. Holter Rythm
The Holter Recorder records heart activity over a period of 24 hours, 48 hours, or sometimes longer. Self-adhesive electrodes are attached to the patient at specific locations on the chest. The electrodes are connected to a small portable recorder. The device is fastened to the belt or worn as a necklace around the neck. It is preferred that the device be applied, and the patient resume his normal daily activities.
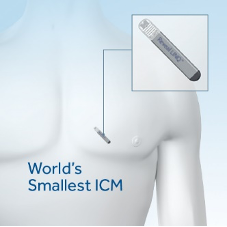
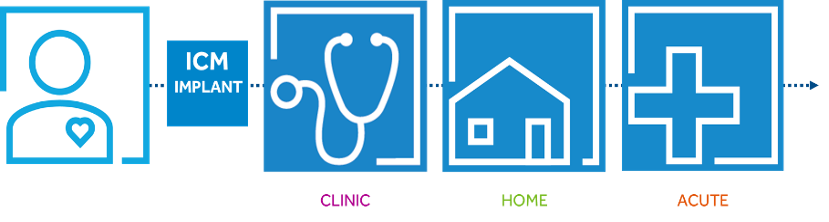
3. Implantable Cardiac Monitoring Devices (ICM-ILR)
Injectable cardiac devices (or ILR Implantable loop recorder or Implantable Cardiac Monitor ICM) are microscopic devices (volume <1.5cc) which are MRI compatible (1.5 and 3.0 Tesla) that are implanted with a very tiny incision under the patient’s skin and continuously record the ECG. They operate on a battery that lasts 2-3 years and can be controlled either by an external programmer or by a device that remotely monitors the patient by the doctor, so that a recorded arrhythmia is retrieved from their memory. These devices are used when the arrhythmias under investigation are rare enough to be recorded during a 24-hour Holter. They can be removed when the device has run out of battery or when the diagnosis has been made.

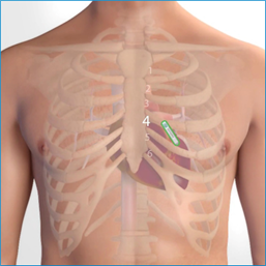

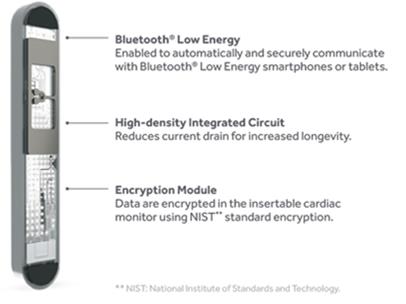
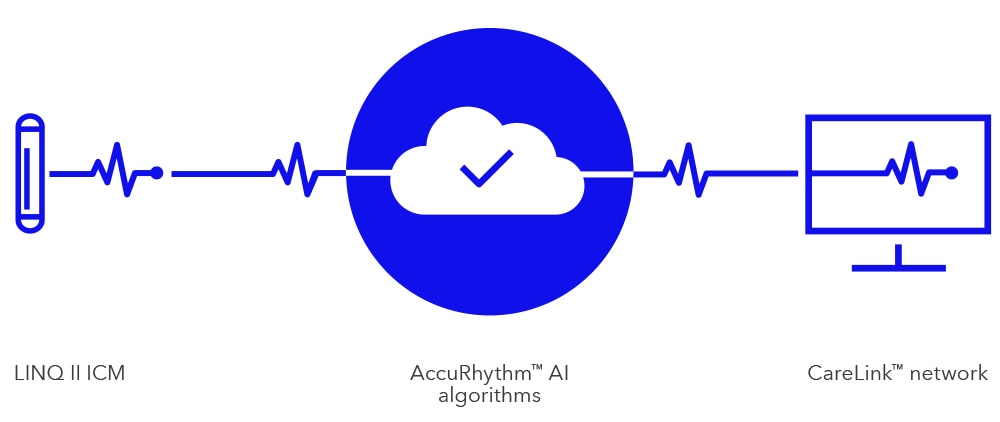
The most advanced AI cardiac monitoring system with the highest diagnostic accuracy in the world according to the most up-to-date published scientific data is now available.
Specifically, the new generation of implantable heart recorders with Artificial Intelligence offers:
a. Unparalleled diagnostic accuracy,
b. Redesigned connectivity,
c. Streamlined workflow
The system in question incorporates the following revolutionary innovations:
a. Artificial Intelligence algorithm to fully minimize false positive arrhythmic events and alerts (Artificial Intelligence)
b. 4.5 years battery life (enhanced battery longevity)
c. complete remote programming by the physician of all detection parameters (complete remote programming)
d. revolutionary early abnormal ventricular contractions detection algorithm to identify high-risk patients (PVC detector)
e. Possibility of self-activation of the initiation of detection by inserting the device into the subcutaneous tissue due to a change in temperature (auto initiated detection)
g. possibility of remote monitoring by the doctor, either through the patient’s outpatient unit, or through a free application through the patient’s mobile phone

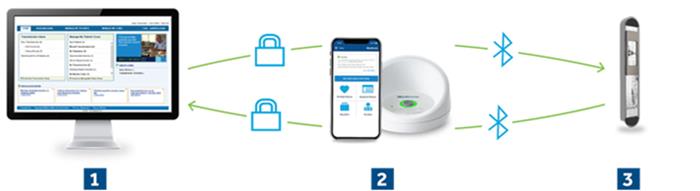
Value of patient remote monitoring:
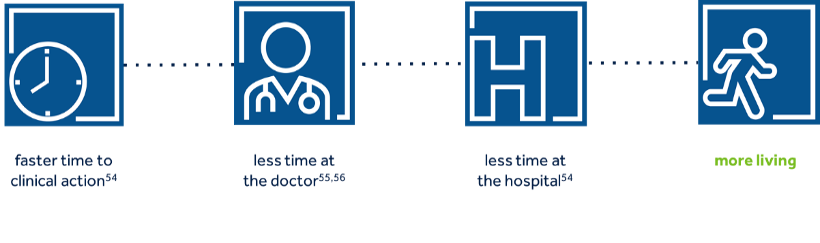
- Routine in-office visits may be replaced by remote visits resulting in 45% fewer[12]in-office visits
- 58% less time [13] for remote vs. in-office follow-up
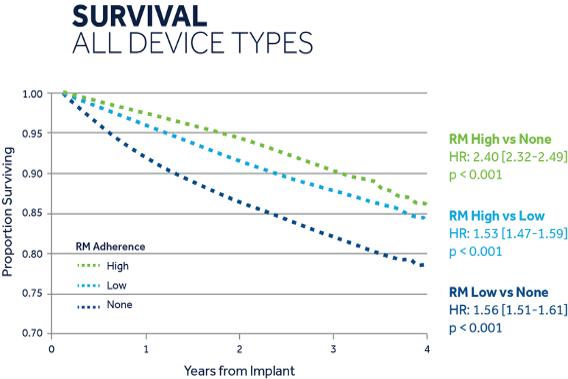
- Patients with high compliance with remote monitoring have been shown to have 53% higher survival rate than patients with low compliance with remote monitoring [14]
Monitoring patients’ diagnostic data can be a challenge but classification is the solution as the monitoring and screening service is designed to help health professionals save time while allowing for better results.
All data are checked by separate certified ECG / cardiac device specialists, under the supervision of cardiologists
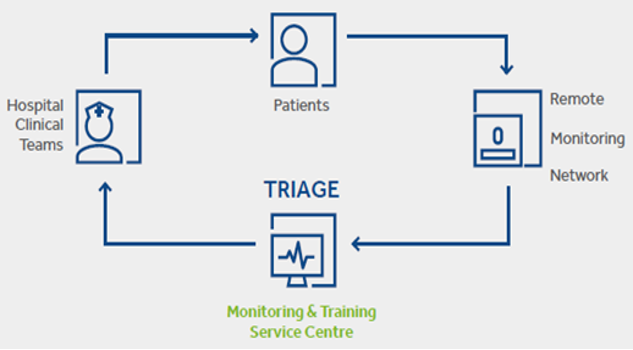
- Freeing up time: Less time spent reviewing nonactionable data means more time for other clinical activities.
80% of transmissions do not require clinical action [12], >3.5h of data review is saved per patient, per year and 3x
faster than standard workflow in reviewing transmissions - Expanding access: Prioritized and proactive communication frees up resources, so more patients can be seen
and treated
Improving quality of the triaging sensing since:
It enables better outcomes: Clinically relevant transmissions are triaged and escalated promptly to hospital clinical teams, allowing patients requiring treatment to be prioritized and treated in a timely manner.
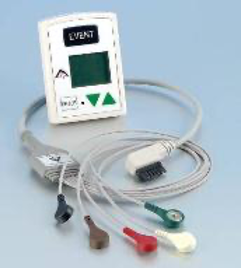
4. Cardiac Event Recorder
Cardiac recorders work like Holter but record when activated by the patient when he feels the specific discomfort being investigated. Other heart rate monitors are activated automatically when they diagnose abnormal heart rhythms based on specific criteria. These devices can accompany the patient for weeks until the abnormal heart rhythm appears.
REFERENCES
[1] Powers WJ, et al. Stroke. 2019;50(12):e344-e418.
[2] http://www.sitsinternational.org/
[3] http://jneurology.gr/images/pdf_2016_1/09.pdf
[4] Tsivgoulis G, et al. Ther Adv Neurol Disord. 2018;11:1756286418783578.
[5] Langhorne P, et al. Stroke. 2000;31(6):1223-1229.
[6] Del Brutto VJ, et al. J Am Coll Cardiol. 2019;74(6):786-803.
[7] Safouris A, et al. Expert Opin Investig Drugs 2021, under press
[8] Saxon LA, et al. Circulation. 2010;122:2359-2367.
[9] Mittal S, et al. Presented at HRS 2014 (LB01-05).
[10] Akar J, et al. Presented at HRS 2014 (LB03-03).
[11] Crossley GH, et al. J Am Coll Cardiol. 2011;57:1181-1189
[12] Cronin EM, et al. Heart Rhythm. 2012;9:1947-1951.
[13] Varma N, et al. Am Heart J. 2007;154:1029-1034
[14] Mittal S, et al. Presented at HRS 2014 (LB01-05).
